
Fig.1. Synthesis of ascidiacyclamide from desoxazoline-ascidiacyclamide.
Desoxazoline ascidiacyclamide derivatives (DOASC) are the precursers of ascidiacyclamide in the organic synthesis.
| Desoxazoline ascidiacyclamide derivatives (DOASC) |
| Ascidiacyclamide (ASC),
cyclo(-Ile-Oxz-D-Val-Thz-)2, is a unique cyclic
octapeptide1 (Fig.1) containing unusual amino acids (Thz, thiazole and
Oxz, oxazoline), and the two major conformations (squared &
folded forms) have been demonstrated by NMR and X-ray diffraction
methods. A conformational equilibrium has been postulated between two
conformers. To control the conformational behavior of ASC in the
equilibrium, we have focused on the C2-symmetry of ASC
and a Ile residue was substituted for Gly, Leu or Phe. The NOE
parameters and molecular dynamics simulations suggested that the
replaced amino acid could be a trigger on shifting the equilibrium
toward the folded conformer. However, the folded conformer has not yet
been captured in solid state in spite of NMR evidences. Our serious
structural studies imply that the Oxz and Thz blocks limit the variation
of molecular folding. Therefore, the release of rigid block(s) would
make the molecules flexible and it seems to be a noteworthy approach to
investigate the structural behaviors of ASC derivatives. We are also
interested in such flexible conformers to consider the control of
molecular folding. Indeed, the desoxazoline-ascidiacyclamide
[(DOASC), 1 =
cyclo(-Ile-aThr-D-Val-Thz-)2], which lacks the
Oxz blocks of ASC, resulted in the novel pseudoboat or pseudochair form
in solution (aThr, L-allo-threonine). Furthermore, the
relationships between DOASC and the accumulation of certain metals in
marine organisms have been suggested and the ability of carrying metals
was assumed for DOASC. Their metal complexes are also interesting in
controlling the conformation.
|

Fig.1. Synthesis of ascidiacyclamide from desoxazoline-ascidiacyclamide. Desoxazoline ascidiacyclamide derivatives (DOASC) are the precursers of ascidiacyclamide in the organic synthesis. |
| We also applied the asymmetric modifications for 1 to pilot the conformer distribution. As shown in folows, the position 1 was replaced with Ala or Val; 2 = cyclo(-Ala-aThr-D-Val-Thz-Ile-aThr-D-Val-Thz-), 3 = cyclo(-Val-aThr-D-Val-Thz-Ile-aThr-D-Val-Thz-), and a epimer of 1 at position 3 was synthesized; 4 = cyclo(-Ile-aThr-Val-Thz-Ile-aThr-D-Val-Thz-). The structures of these compounds were analyzed by X-ray diffraction method. |
compound iasco aasco vasco ilvasco Formula 2(C36H56N8O8S2) C33H50N8O8S2 2(C35H54N8O8S2) 2(C36H56N8O8S2) Solvent(s) H2O CH3CH2OH 2(CH3CH2OH) 4(C3H8O)�H2O Mr 1604.0 797.0 704.1 1844.4 Crystal system monoclinic orthorhombic monoclinic monoclinic Space group P2 P212121 P21 P21 a (A) 17.884(10) 18.6011(3) 13.9298(3) 15.4631(299) b (A) 11.225(6) 20.2838(3) 18.7115(3) 16.5630(196) c (A) 25.41(2) 10.4905(2) 16.2356(4) 20.8925(454) b (�) 93.90(6) 90.0 91.0671(7) 109.8615(875) Volume (A3) 5089(6) 3958.08(11) 4231.03(15) 5033(16) Z 4 4 4 4 Temperature (K) 273(2) 100(2) 100(2) 100(2) Description plate cubic plate needle Size (mm3) 0.40x0.25x0.10 0.12x0.12x0.12 0.30x0.12x0.08 0.30x0.02x0.02 Dx (g cm-1) 1.047 1.337 1.295 1.212 F(000) 1716 1704 1768 1972 m (mm-1) 1.350 0.197 0.187 0.166 Radiation source CuKa SPring-8* SPring-8* SPring-8* Wavelength (A) 1.5418 0.834 0.834 0.834 NREF 5981 7969 5590 10097 Resolution (A) 0.97 0.80 0.80 0.80 Structure sol. SHELXS-97 SHELXS-97 SHELXS-97 LODEM Refinement SHELXL-97 SHELXL-97 SHELXL-97 SHELXL-97 Npara 983 498 1031 1127 R1 0.0989 0.0687 0.0510 0.0574 wR 0.2768 0.1863 0.1486 0.1545 (shift/d)ma 0.222 0.008 0.115 0.348 Drmax (e A3) 0.507 0.828 0.358 0.615 Drmin (e A3) -0.422 -0.566 -0.367 -0.413 Get PDB yes yes yes yes

[Gly]DOASC |

[Ala]DOASC |
|

[Val]DOASC |
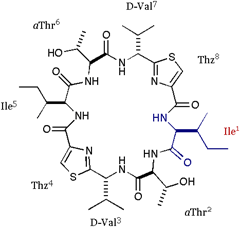
[Ile]DOASC (parent) |
|

[Leu]DOASC |

[Phe]DOASC |
|
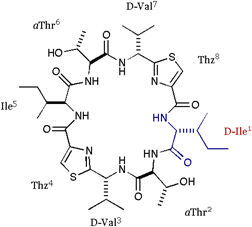
[D-Ile]DOASC |

[Ile1,Val3]DOASC |
|

[Aib]DOASC (Aib: amino-iso-butylic acid) |
|